Research
The Dias Lab is broadly interested in two areas 1)human genetics and 2)neurogenetic mechanisms of human brain development and degeneration. We are interested in better understanding the fundamental genetic and molecular mechanisms that make us uniquely human.
Human Genetics:
We study fundamental genetic mechanisms, such as the role that short repetitive genomic elements, play in the human condition. We pilot challenging techniques directly in primary human cells and tissues, such as long read sequencing and single nucleus sequencing. In addition to these cutting-edge approaches, we strive to develop methodologies that do not require specialized expertise or equipment, in order to accelerate study of similar questions more broadly within the scientific community.
Genetic Disorders
We also study genetically defined disorders, such as Fragile X syndrome and Fragile X-associated tremor/ataxia syndrome. We seek to characterize pathophysiology that underlie these uniquely human disorders. We focus on questions historically understudied, such as the role of the X chromosome and non-neuronal cell types in these disorders. We harness developmental context as a critical biological variable that impacts the expression of powerful germ-line genetic drivers.
Cellular Heterogeneity:
The brain is an amazingly complex and heterogeneous tissue and is not immediately accessible for study in the human condition. We are interested in studying the molecular perturbations associated with neurodevelopmental and neurodegenerative disorders in different cellular subtypes in the human brain specifically. In order to do this, we are applying single cell genomic approaches on post-mortem human brain to identify cell-type and region-specific molecular changes related to brain function.
Collaborations:
Collaborative efforts are the only path forward to conduct truly transformative science. We collaborate with laboratories within and outside of our department and university. Within our laboratory, collaborative projects are the norm, and not the exception. We are always looking for new opportunities to develop creative lines of investigation.
Highlighted

All
2023

2022
2021
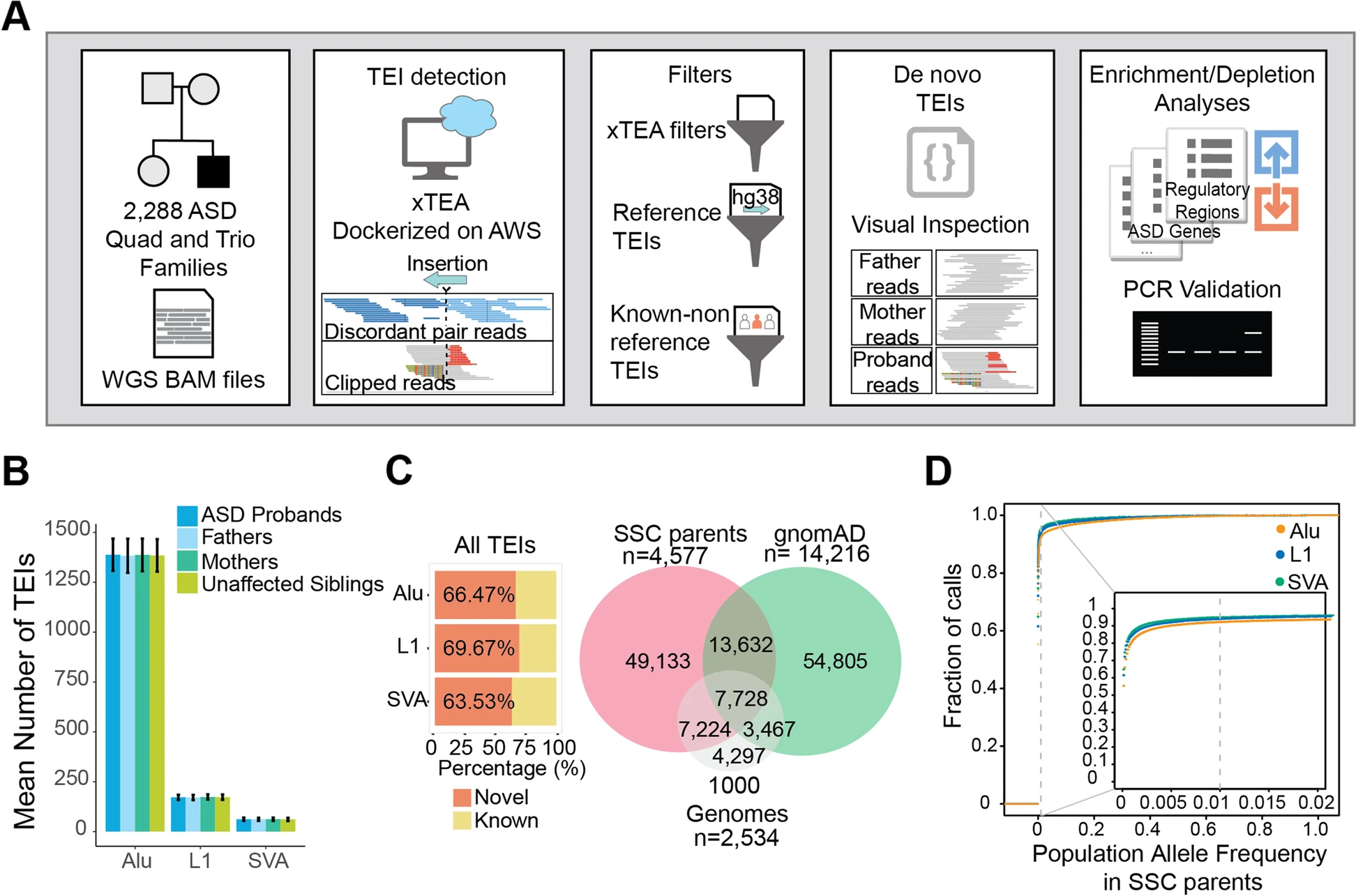
2020


2019

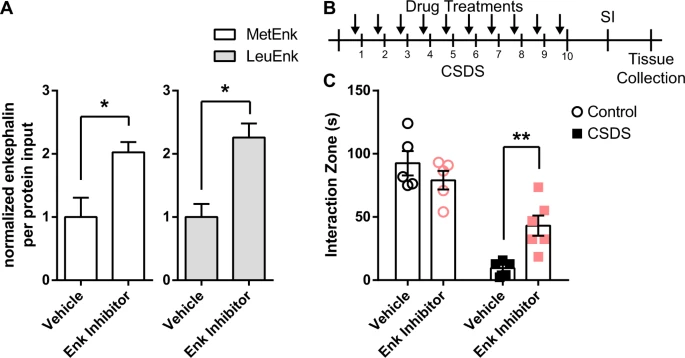
2017
2016


2015
2014
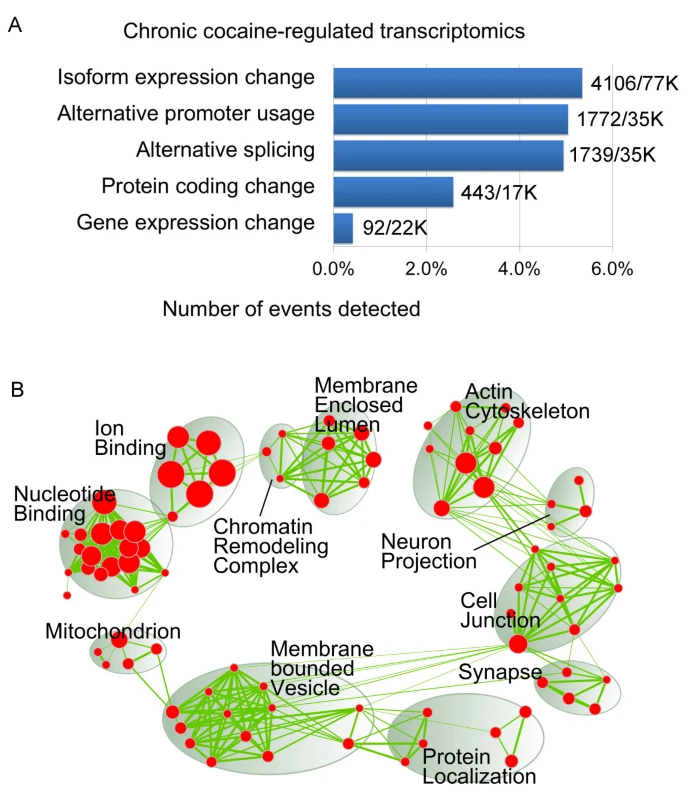
2013

2012

2011

2008